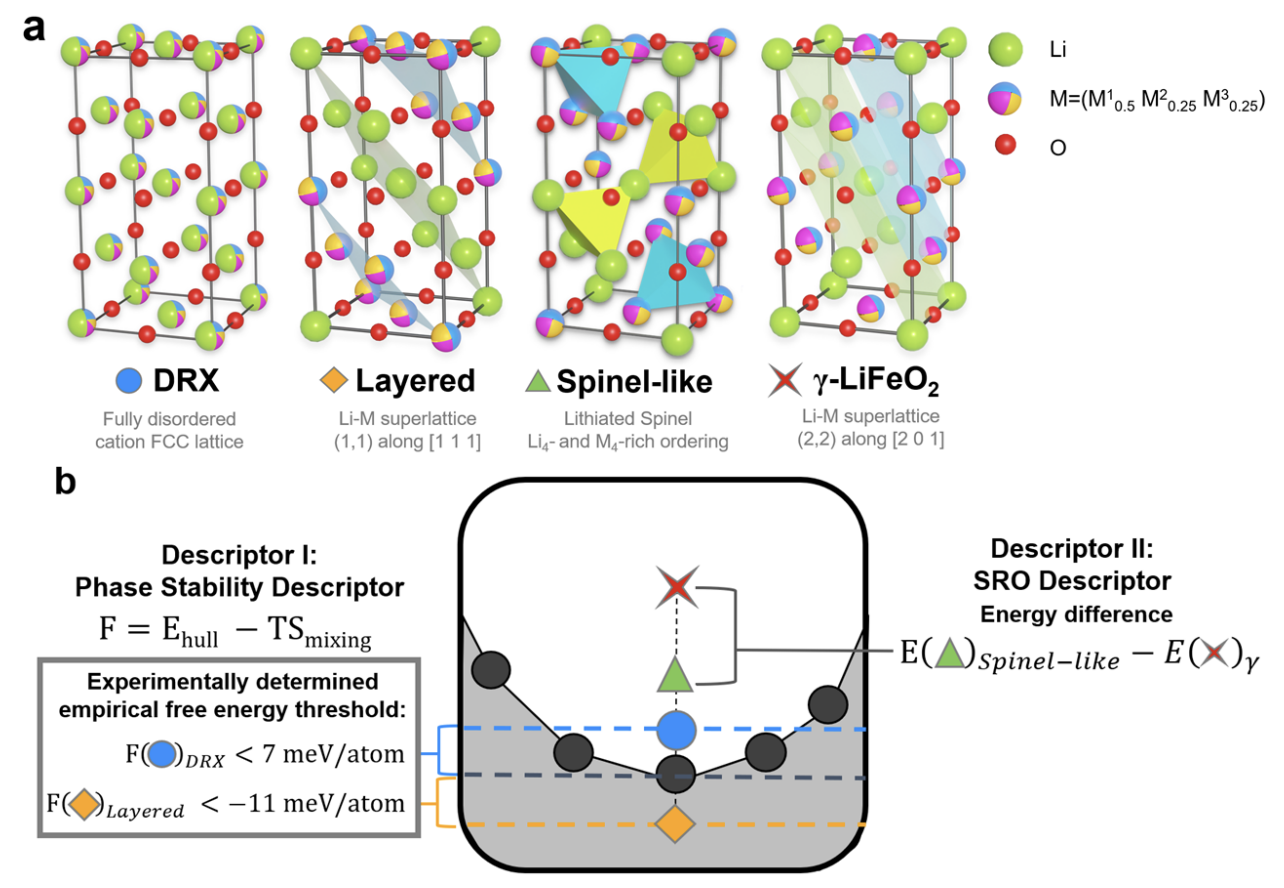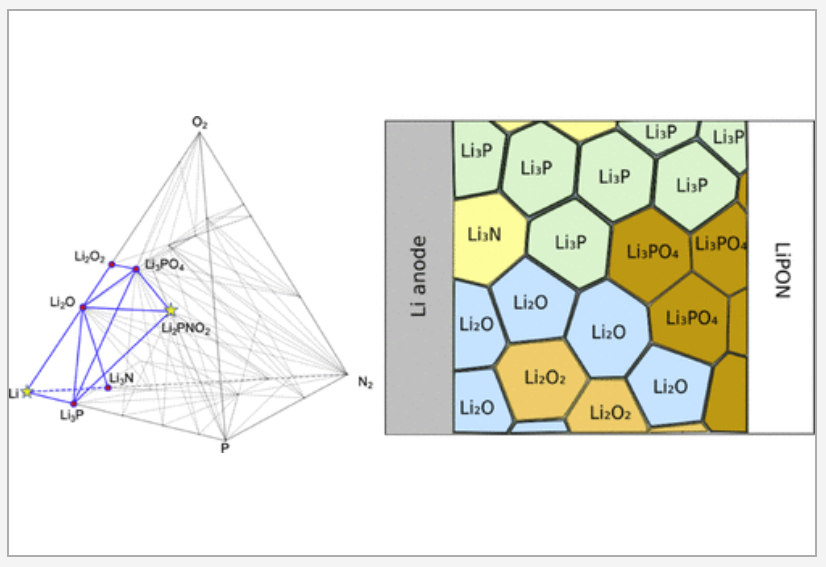
All-solid-state lithium-ion batteries have attracted significant research interest for providing high power and energy densities with enhanced operational safety. Despite the discoveries of solid electrolyte materials with superionic conductivities, it remains a challenge to maintain high rate capability in all-solid lithium-ion batteries in long-term operation. The observed rate degradation has been attributed to reactivity and resistance at the electrode–electrolyte interfaces. We examine interfaces formed between eight electrolytes including garnet, LiPON, and Li10GeP2S12 (LGPS) and seven electrode materials including an NCM cathode and a metallic Li anode and identify the most rapid lithium-ion diffusion pathways through metastable arrangements of product phases that may precipitate out at each interface. Our analysis accounts for possible density functional theory (DFT) error, metastability, and finite-temperature effects by statistically sampling thousands of possible phase diagrams for each interface. The lithium-ion conductivities in the product phases at the interface are evaluated using machine-learned interatomic potentials trained on the fly. In nearly all electrode–electrolyte interfaces we evaluate, we predict that lithium-ion conduction in the product phases making up the interphase region becomes the rate-limiting step for battery performance. READ MORE